- Seen : 552 View
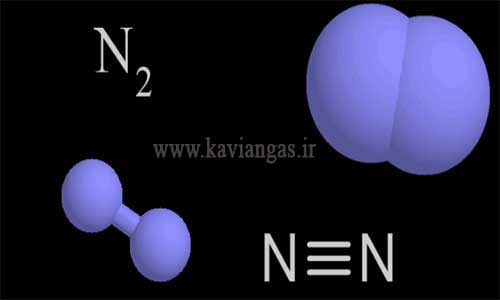
Nitrogen gas is abbreviated as N, whose atomic number is 7 in the periodic table. Nitrogen gas can be considered the main element in the atmosphere and living organisms. Usually, nitrogen in the air (free) is a colorless, odorless and tasteless gas. That is why this gas is included in the category of inert gases.
Nitrogen gas in the air reduces the amount of oxidizing gases, thus preventing spontaneous burning and corrosion of metals. There are important molecular structures in the body of living organisms, such as amino acids, proteins, and nitrogen forms part of these structures. Nitrogen gas has many applications in the industry, including it can replace air in food packaging. Ammonia is also a nitrogen compound that is used in chemical fertilizers and nitric acid production. It should be mentioned that nitrogen is also used in the production of gunpowder and TNT bombs.
Nitrogen gas comprises about 78% of air volume and 5% of air mass. The most common isotope of nitrogen is known as nitrogen-14, which is produced by nuclear fusion in stars.
Preparation of nitrogen gas
One of the nitrogen production methods is the COLD BOX method. In this production method, nitrogen with a purity of 99.99% is usually produced and prepared. In fact, it is possible to produce pure nitrogen for purposes that require high purity. It should be mentioned that liquid nitrogen can only be prepared by this method.
The stages of producing nitrogen gas by the COLD BOX method:
First, the temperature of the incoming compressed air is reduced by 10 degrees by the water chiller, and it enters the next stage, the drive, where the impurities, oil and moisture are taken and dried, and when it enters the COLD BOX, its temperature is reduced to 196 degrees Celsius and becomes becomes liquid. This liquid air that has been produced enters the lower part of the tower, and with the difference in the dew point of oxygen and nitrogen, nitrogen comes out first and then oxygen.
Unique physical and chemical properties of nitrogen gas:
As you already know, nitrogen gas is in the fifth group of the periodic table, and in fact, each house of the periodic table has its own properties, so we decided to provide you with information to identify the physical and chemical properties of nitrogen gas. give:
Nitrogen gas has an atomic number of 7 and an atomic mass of 14.00674. The melting point and boiling point of nitrogen gas are -210.0 and -195.7, respectively. Nitrogen has an atomic radius of 0.75 Angstroms. Since nitrogen gas is in group 5 of the periodic table, it has a capacity of 5. Nitrogen gas is a colorless gas and is in its normal and standard state as a gas.
Nitrogen gas has an ionization energy of 1402.3 kJ/mol. The electronic form of this gas is 1S2 2S2 2P 3. Nitrogen gas has several stable and semi-stable isotopes, the two stable isotopes of nitrogen gas are N-14 and N-15.
Nitrogen gas detection methods:
There are two ways to identify nitrogen gas: the first method is to use a catharometer, in which the type of gas is determined by measuring the thermal conductivity of the gas. The second method is to use red litmus paper, which you have to moisten and put inside the test tube and then fill the test tube with the desired unknown gas. If the paper changes color to blue, it indicates that the gas has the property of play. If its color turns red, it means that it is an acid gas, and if it does not undergo any color change, it means that there is an elemental gas like nitrogen inside the test tube, which can be determined by a flame test that this gas is oxygen or nitrogen.
Regarding the explosion of nitrogen gas, it should be said that this gas is not flammable or explosive. But if its tanks are exposed to fire and high temperature, there is a possibility of bursting. It should be mentioned that nitrogen gas is used in the food industry to prevent spoilage caused by oxidation, mold or insects.
Application of nitrogen:
Another widely used and important gas in human life and other living organisms is nitrogen gas. Here we describe a series of important uses of nitrogen.
If we want to talk about the use of nitrogen in the industry, the use of nitrogen gas for drying is an additional solvent, which is one of the research methods.
Nitrogen is also widely used in the pharmaceutical industry, which uses refined nitrogen in the composition and storage of divided medical mixtures, that's why nitrogen gas is very vital in the pharmaceutical industry.
In the food industry, the gas that is effective for moving air in food packaging is nitrogen gas, this reduces food spoilage, so nitrogen gas is also useful and important in the food industry.
Another use of nitrogen is to produce metals that need to be produced in an environment where there is no oxygen in that environment to prevent oxidation and the production of different metals, therefore nitrogen is used.
Another application of nitrogen gas is in the oil industry, where nitrogen (nitrogen) is used as an important element in the separation of gases and their preparation for oil storage; Because nitrogen does not react with oil.
It should be mentioned that in the new era, instead of filling passenger car tires with air, they use nitrogen gas, the use of nitrogen in tires reduces fuel consumption, increases safety, increases the durability of rims, etc. and what is more interesting is that by using You don't need to check the tire pressure for several months due to nitrogen gas in the tire.
Nitrogen safety tips:
It should be mentioned that the most basic point regarding the nitrogen gas cylinder that is used in a compressed form is to pay attention to the blur
Saler Company Information










